GCSE Science | Energy Changes
What is an exothermic reaction?
When energy is released to the surroundings
What is an endothermic reaction?
When energy is absorbed from the surroundings
What is activation energy?
The minimum energy needed to start a reaction
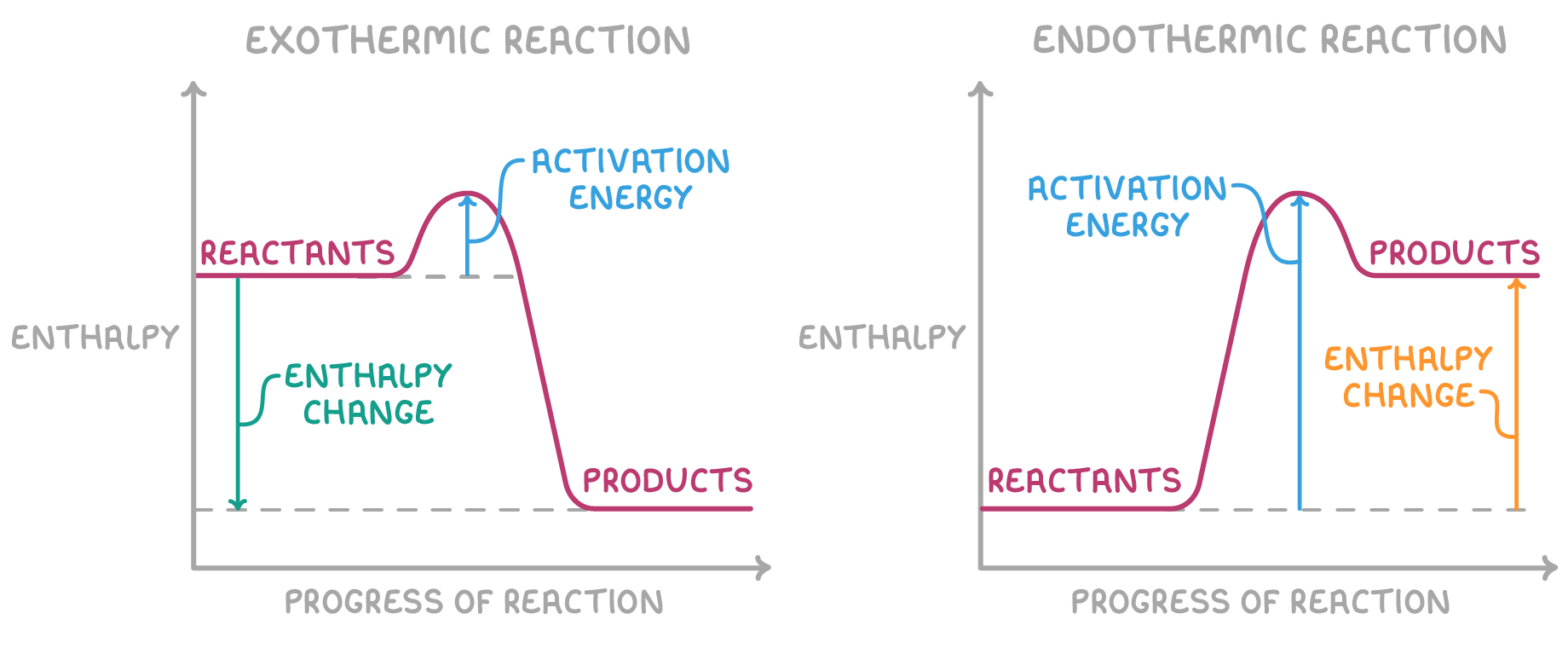
What to label on an energy change diagram?
- y-axis: energy
- x-axis: progress of reaction
- reactants
- products
- activation energy
- energy change
Reaction profile diagrams
How do we calculate energy change?
- add up all the energy needed to break the reactant bonds
- add up all the energy released from forming the product bonds
- energy change = reactants - products
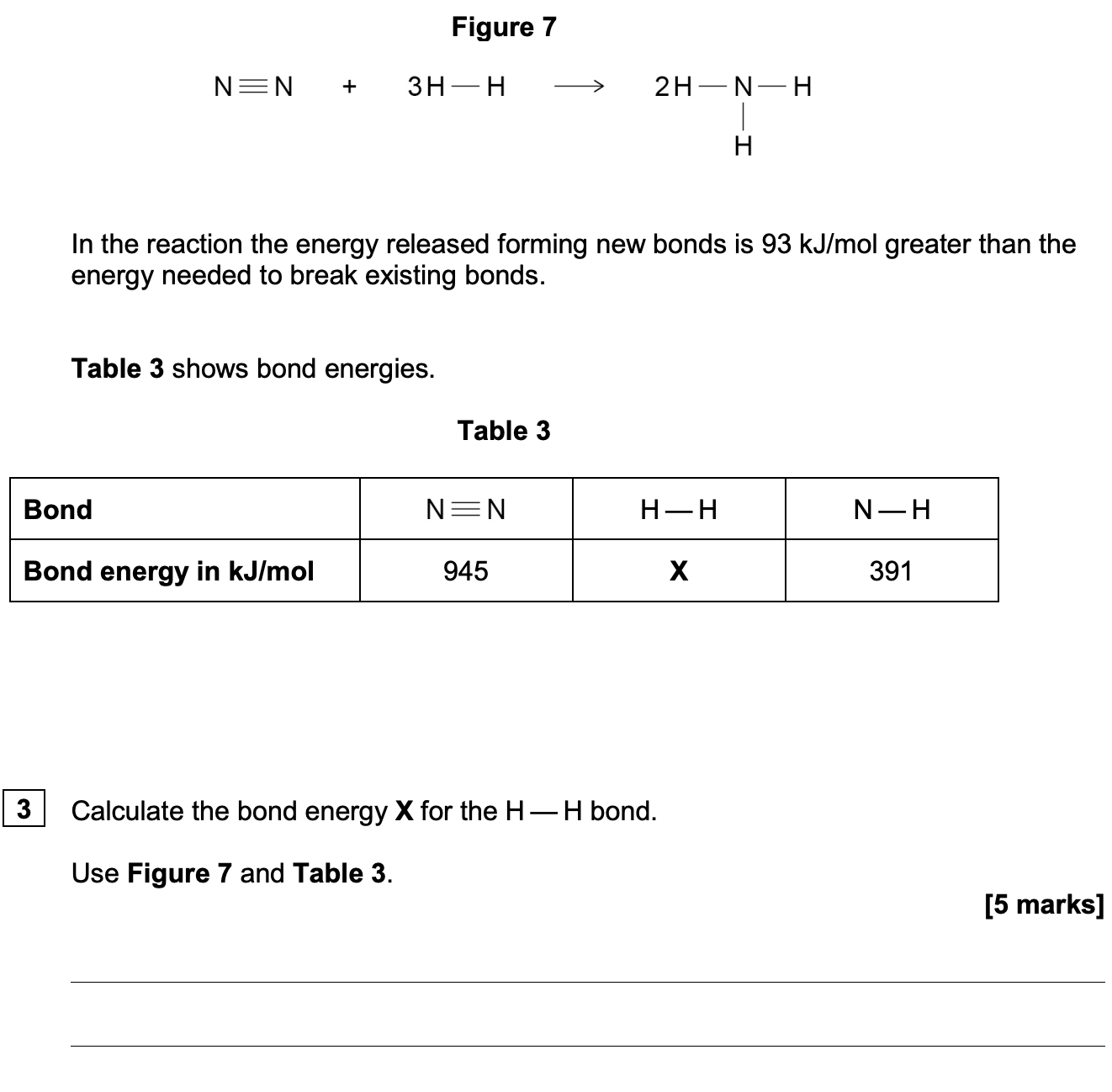
Question 1
Calculate the energy change for the reaction and state whether the reaction is exothermic or endothermic.
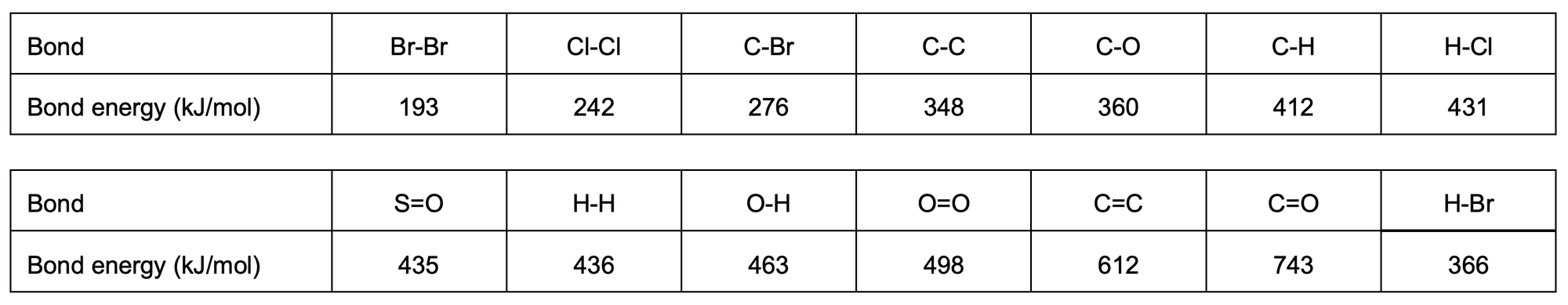
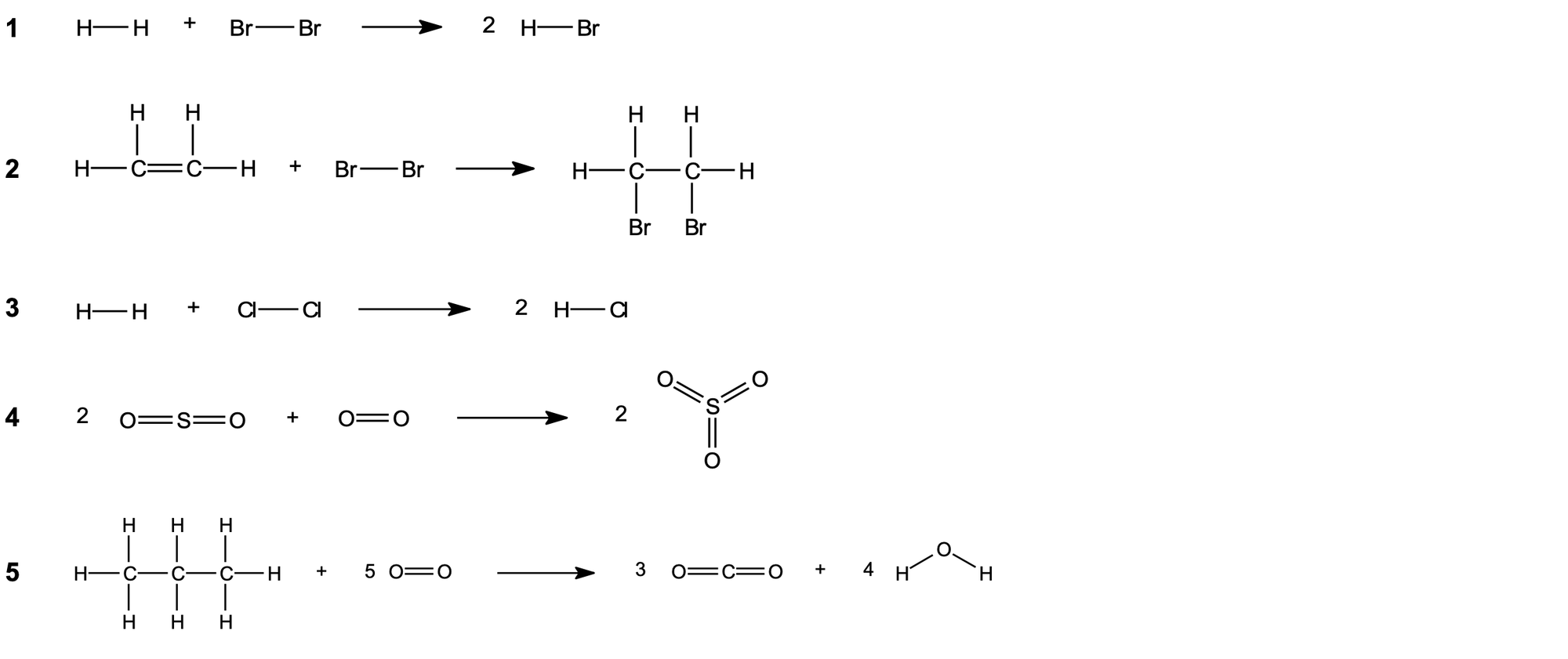
Question 2