GCSE Science | Structure and Bonding
Ionic bonding
- between a metal and a non-metal
- the electrostatic attraction between a metal and a non-metal ion
Examples of ionic structures
- sodium chloride, sodium oxide, magnesium chloride, aluminium oxide
Diagram
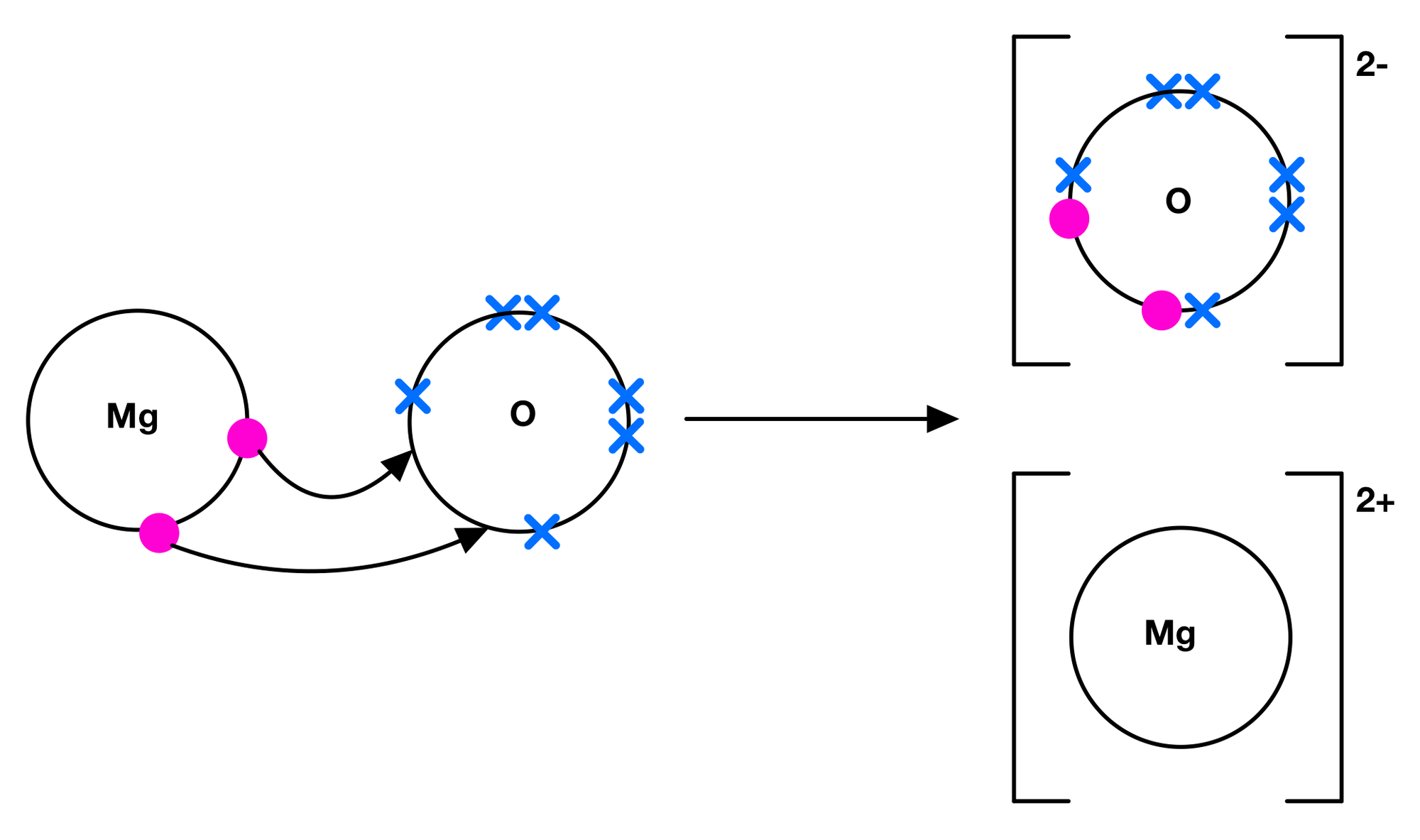
Explanation in terms of ions and movement of electrons
- Magnesium loses two electrons
- to become a 2+ ion
- Oxygen gains two electrons
- to become a 2- ion
- There is electrostatic attraction between the opposite ions.
Properties
- high mp/ bp
- strong electrostatic attraction between opposite ions
- which requires a lot of energy to overcome
- it conducts electricity when molten
- ions are free to move
- so they can carry charge
- it doesn't conduct electricity when solid
- ions are not free to move
- so they cannot carry charge
Covalent bonding
- between non-metal and non-metal
- a shared pair of electrons
Examples of simple covalent molecules
- carbon dioxide, oxygen, water, nitrogen oxide
Diagram
Properties of a simple covalent molecule
- low mp/ bp
- weak intermolecular forces which
- requires less energy to overcome
- does not conduct electricity
- there are no delocalised electrons that are free to move
- not able to carry charge
Examples of giant covalent structures
- diamond, graphite, graphene, fullerenes
Structure of giant covalent structures
- all of these are made of carbon atoms covalently bonded to each other
- diamond - each carbon atom is bonded to 4 other carbon atoms
- graphite - each carbon atom is bonded to 3 other carbon atoms
- graphene - same as graphite, but only one single layer
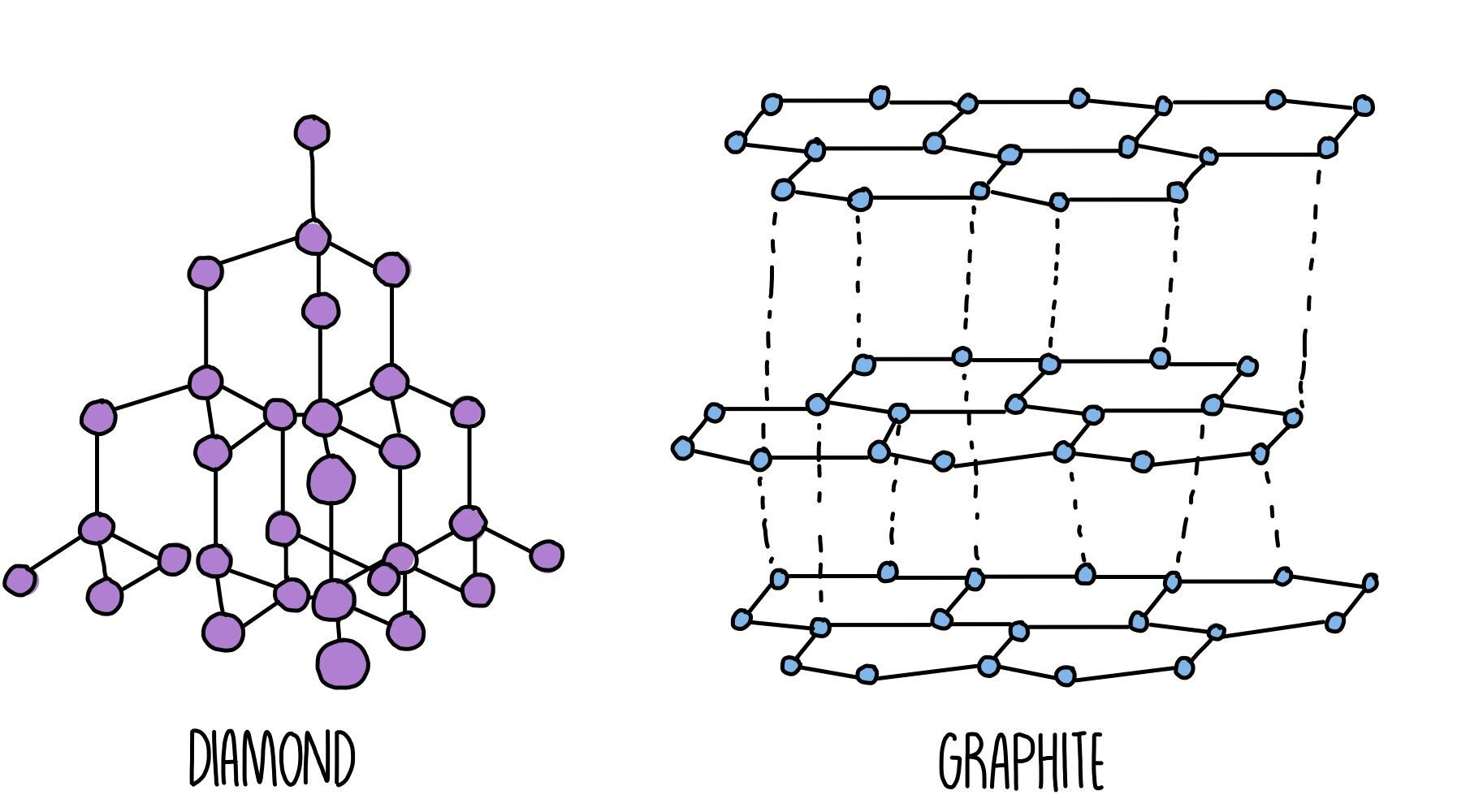
Properties of giant covalent structures
- high mp/ bp
- lots of strong covalent bonds
- lots of energy required to overcome
- diamond does not conduct electricity
- no delocalised electrons
- not free to move
- cannot carry charge
- graphite/ graphene does conduct electricity
- delocalised electrons
- free to move
- can carry charge
- graphite is slippery
- weak intermolecular forces between layers
- layers can slide over each other
Metallic bonding
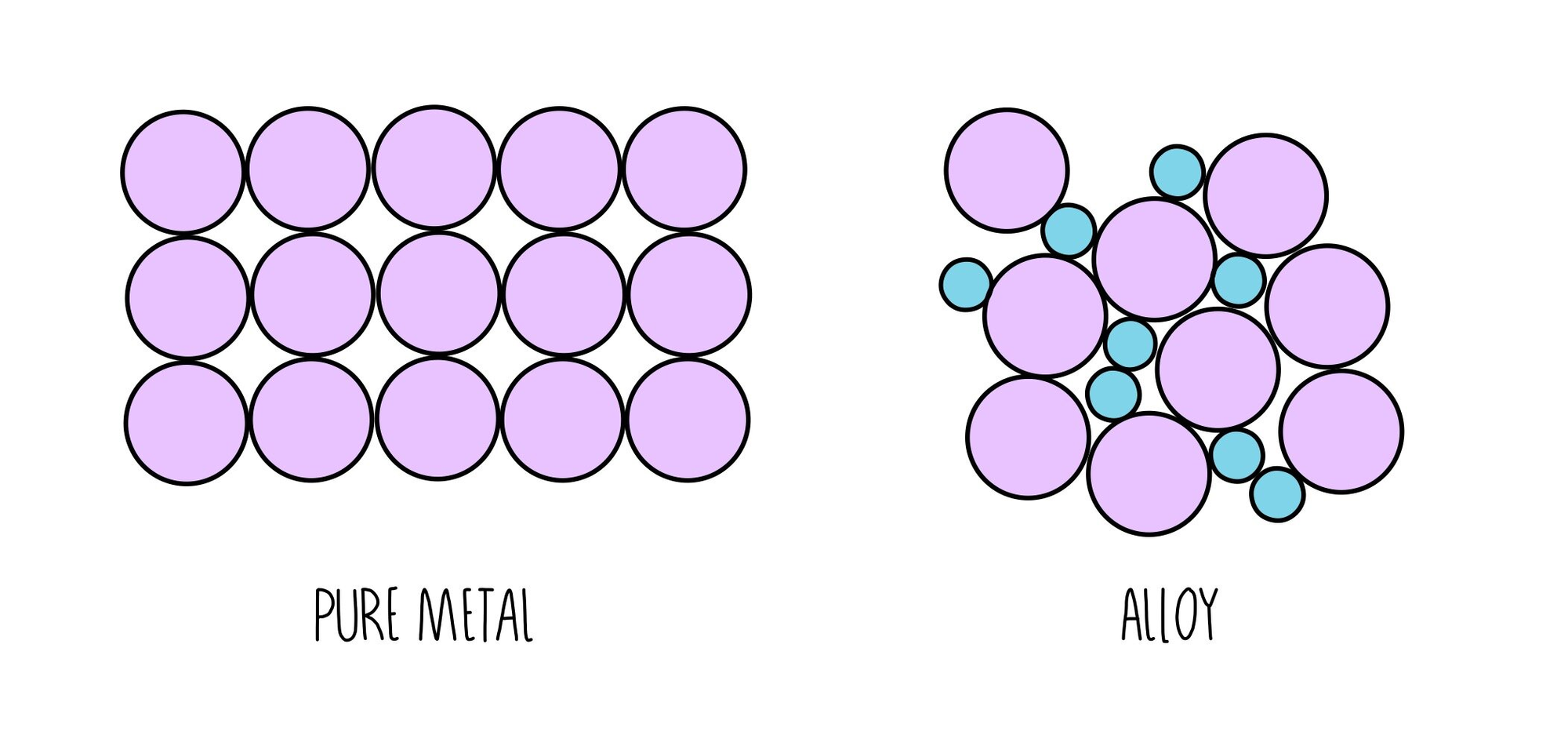
Alloys
- mixture of two or metals
- harder than pure metals
- particles are different sizes
- particles are not arranged in neat rows and columns
- cannot slide over each other if hammered
Triple Science Only
Nanoparticles
- very high surface area to volume ratio
- useful for catalysts, medicine, electronics
Risks of using nanoparticles
- can be breathed in/ enter cells and catalyse harmful reactions
- toxic substances can easily bind to them due to large surface area per cm3
- recent development so lack of scientific research