GCSE Science | Periodic Table
Last updated on
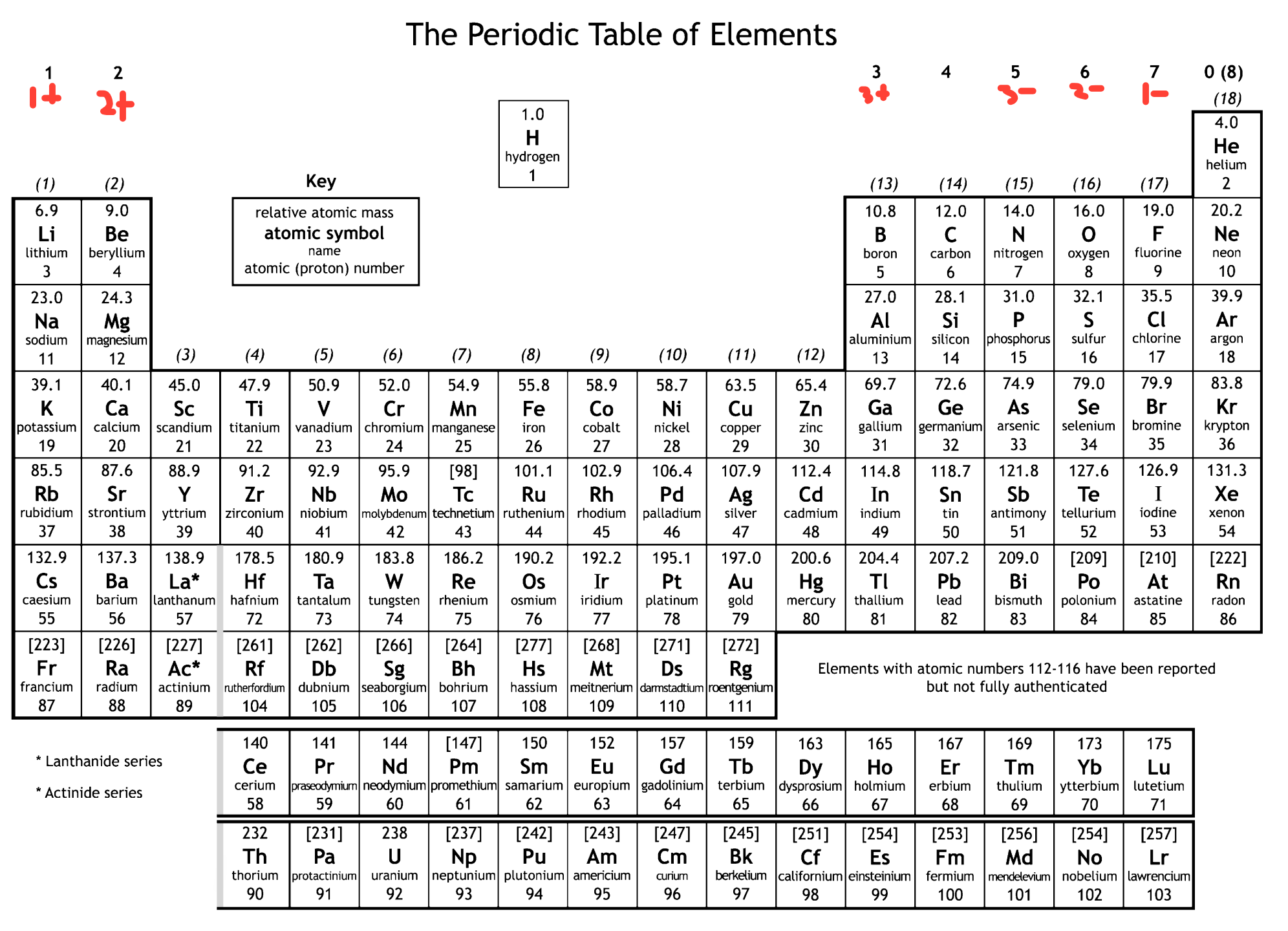
Group Number
- the columns (1, 2, 3, ... 8)
- the number of electrons in the outer shell
- elements in the same group have similar chemical properties
Why do things react?
- elements want to gain a full or an empty outer shell
Group 8
- noble gases
- they already have a full outer shell
- very unreactive
Ions
- Ions are formed when an atom
- gains or loses electrons
Examples of ions
- group 1 elements try to lose 1 electron
- forming 1+ ions
- group 7 elements try to gain one electron
- forming 1- ions
How does reactivity change as you go down group 1? (3 marks)
- reactivity increases as you go down group 1
- easier to lose an electron
- outer shell further away from the nucleus
- more electron shielding
- less nuclear attraction
How does reactivity change as you go down group 7? (3 marks)
- reactivity decreases as you go down group 1
- harder to gain an electron
- outer shell further away from the nucleus
- more electron shielding
- less nuclear attraction
