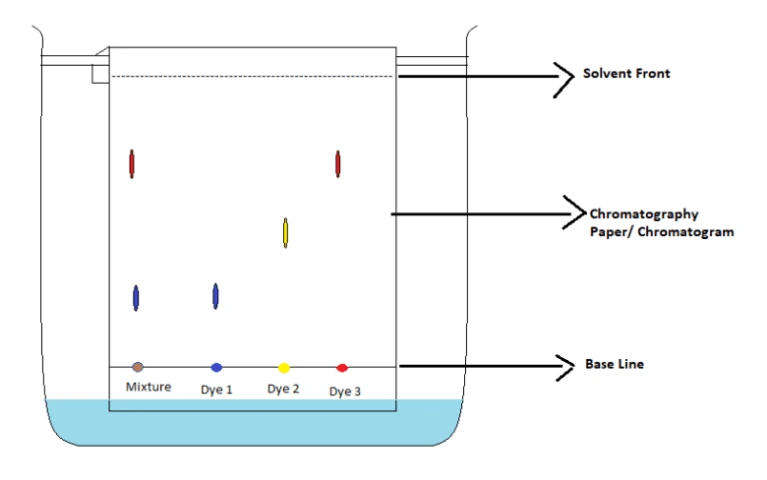
GCSE Science | Paper Chromatography
Pure compounds vs mixtures
- The compounds in a mixture may separate into different spots
- a pure compound will produce a single spot in all solvents.
Paper chromatography
- used to sepearate mixtures
Stationary phase
- doesn't move
- paper
Mobile phase
- moves
- water or ethanol
Method
- the baseline is drawn with a pencil
- the mixture is spotted onto the baseline
- dip the bottom of the paper into a solvent
- each part of mixture has different relative attraction to the stationary and mobile phases, so travels up by a different amount
https://www.tuttee.co/blog/chem-paper-chromatography
Rf value
- distance moved by the substance/ distance moved by the solvent
- always between 0 and 1