GCSE Science | Ionic Bonding
What does the group number tell you?
The group number tells us the number of electrons in the outer shell
How many electrons do elements want on their outer shell?
All elements want to have a full outer shell.
What is an ion?
An ion is a charged particle
What is a positive ion?
A positive ion is an atom that has lost an electron.
What is a negative ion?
A negative ion is an atom that has gained an electron.
Is the melting point and boiling point of ionic compounds high or low?
- high mp/bp
- strong electrostatic attraction between opposite ions.
- this requires a lot of energy to overcome


Draw and explain the bonding in barium oxide
1) barium oxide (Ba and O) Formula: _______________
Diagram:
Draw and explain the bonding in sodium chloride
2) sodium chloride (Na and Cl) Formula: _______________
Diagram:
Draw and explain the bonding in sodium bromide
3) sodium bromide (Na and Br) Formula: _______________
Diagram:
- sodium loses one electron
- and forms a 1+ ion.
- bromine gains an electron
- and forms a 1- ion.
4) calcium chloride (Ca and Cl) Formula: _______________
Diagram:
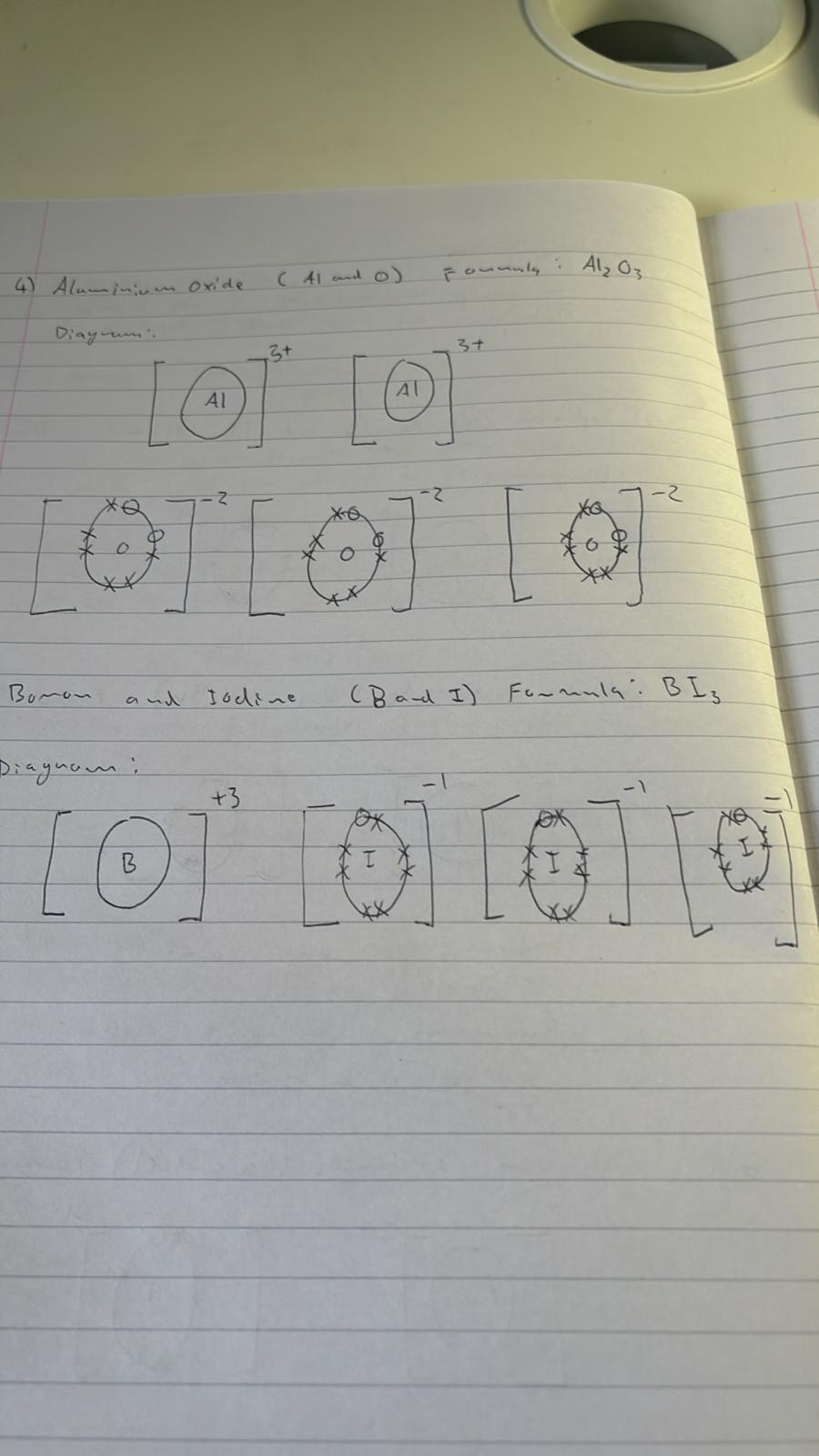
5) aluminum oxide (Al and O) Formula: _______________
Diagram:
6) sodium oxide (Na and O) Formula: _______________
Diagram:
7) sodium nitride (Na and N) Formula: _______________
Diagram:
8) magnesium phosphide (Mg and P) Formula: _______________
Diagram: