GCSE Science | Concentration
- concentration = moles/ volume
- concentraion: mol/dm3
- moles: mol
- volume: cm3
- 1cm3 = 0.001dm3 (divide by 1000)
Question 1
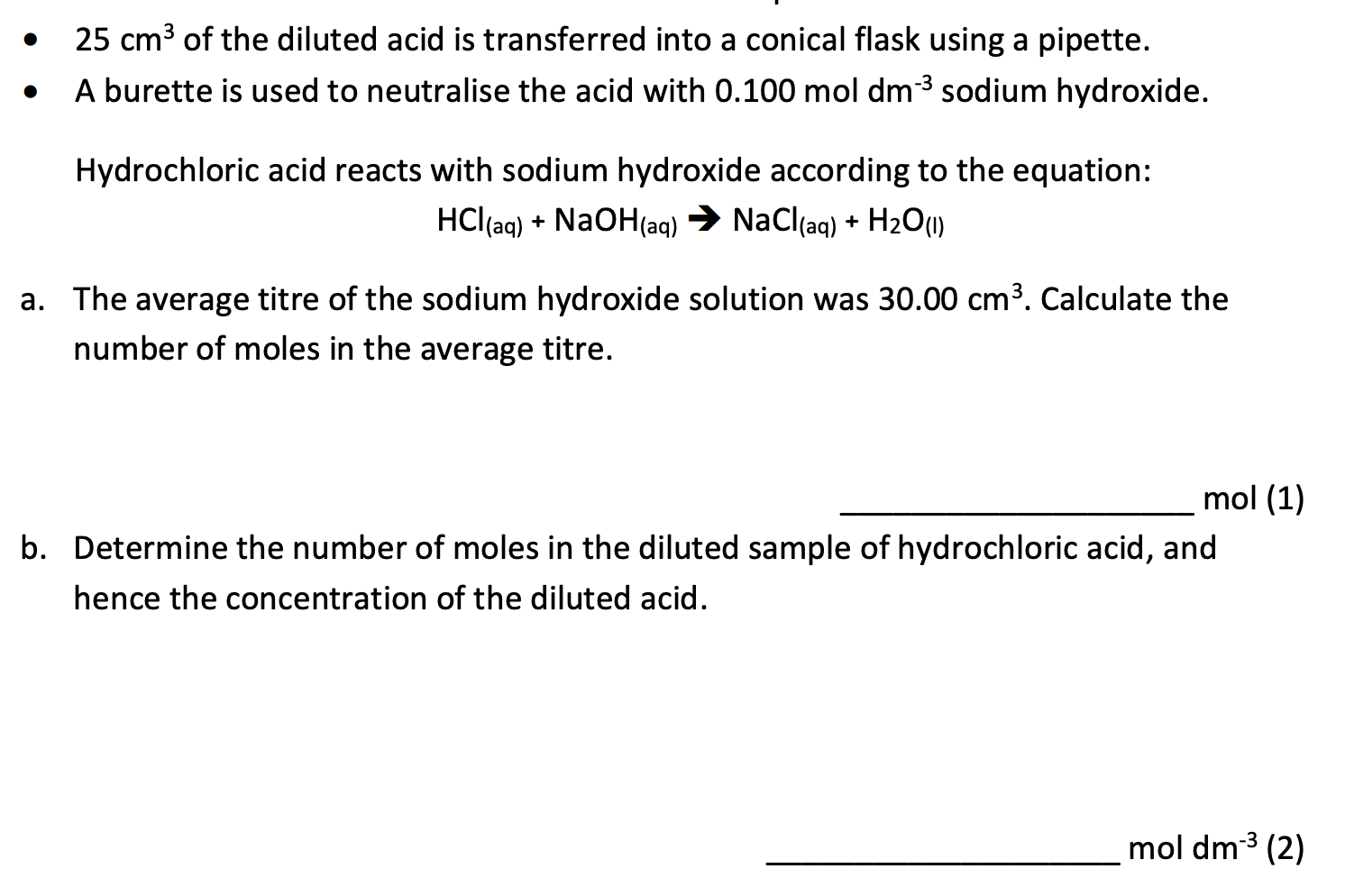
Question 2
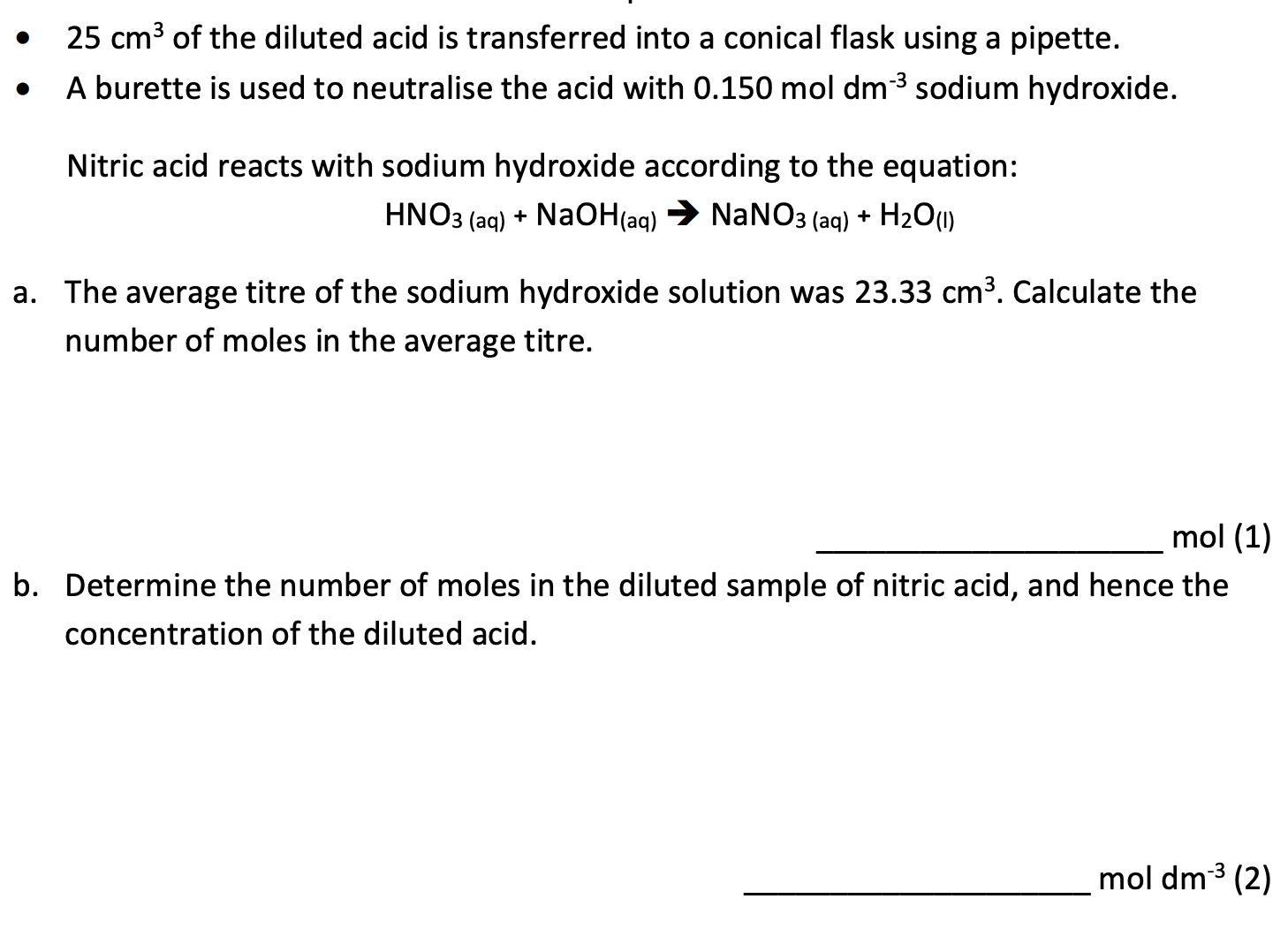
Question 3
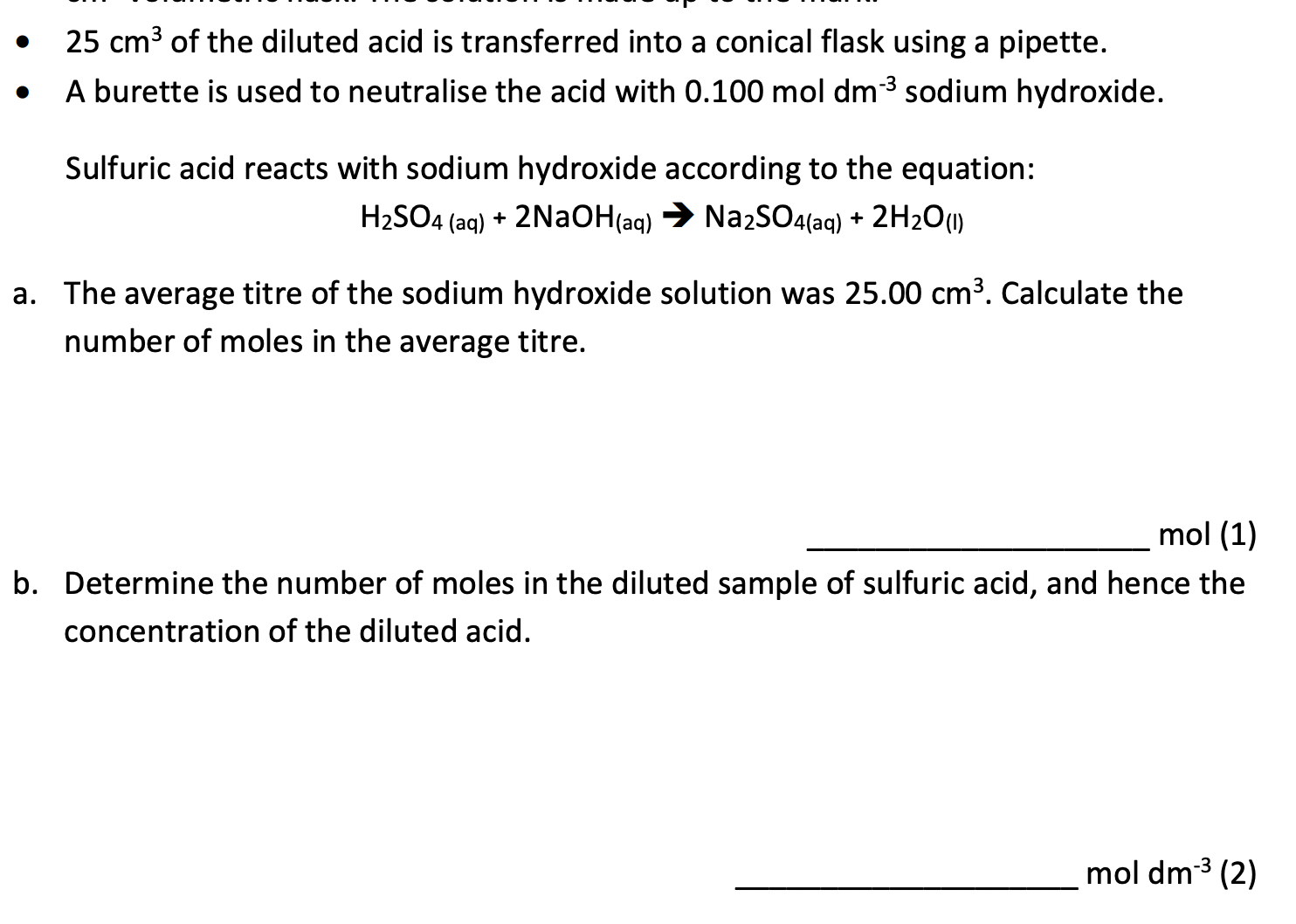
Volume of gases at room temperature and pressure
- at room temperature and pressure, 1 mole of gas occupies 24 dm3
- the concentration of gas at r.t.p is 1/24 mol/dm3
Question 4
